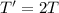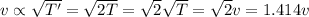Chemistry, 04.04.2020 06:46 brendariobranco
If the absolute temperature of a gas is doubled, what happens to the root‑mean‑square speed of the molecules? Nothing happens to the rms speed. The new rms speed is 4 times the original rms speed. The new rms speed is 2 times the original rms speed. The new rms speed is 1.414 times the original rms speed. The new rms speed is 1/2 the original rms speed.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
If the absolute temperature of a gas is doubled, what happens to the root‑mean‑square speed of the m...
Questions

Geography, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29

Physics, 03.04.2020 02:29




Biology, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29



Computers and Technology, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29

Spanish, 03.04.2020 02:29

English, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29


Mathematics, 03.04.2020 02:29

Mathematics, 03.04.2020 02:29

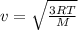
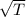
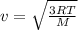
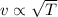 (1)
(1)