Hc2h3o2(aq) + ba(oh)2(aq) + > h2o(l) + ba(c2h3o2)(aq)
balance this equation and determine...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 09:30
The mass of a proton is approximately equal to the mass of
Answers: 1
You know the right answer?
Questions

Mathematics, 12.08.2020 05:01




Mathematics, 12.08.2020 05:01








Mathematics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01



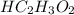
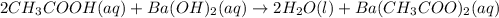
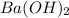
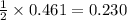 moles of
moles of 


