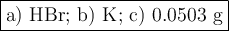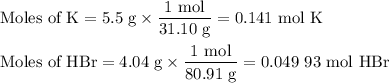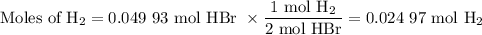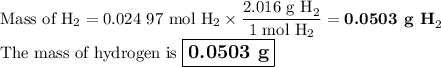2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hyd...

2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
2K + 2HBr → 2 KBr + H2
When 5.5moles of K reacts with 4.04moles of HBr, to produce Hydrogen gas(H₂)
●a). What is the limiting reactant?
●b.)What is the excess reactant?
●C.)How much product is produced?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

You know the right answer?
Questions


Mathematics, 04.11.2020 22:00



History, 04.11.2020 22:00

Advanced Placement (AP), 04.11.2020 22:00

Chemistry, 04.11.2020 22:00

Spanish, 04.11.2020 22:00

Business, 04.11.2020 22:00

Social Studies, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00


Spanish, 04.11.2020 22:00


History, 04.11.2020 22:00

Mathematics, 04.11.2020 22:00


Social Studies, 04.11.2020 22:00