

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1
You know the right answer?
If 2.87 g of aluminum are reacted with excess copper (II) sulfate and 9.2 g of copper are produced w...
Questions

History, 10.03.2020 22:38


Mathematics, 10.03.2020 22:38





History, 10.03.2020 22:38


Mathematics, 10.03.2020 22:39










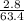
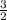 =
=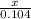
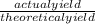 x 100
x 100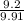 x 100
x 100

