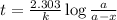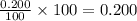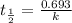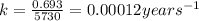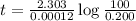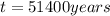Chemistry, 03.04.2020 05:02 jayrichesz80Jahree
The half-life of radioactive carbon-14 is 5730 years. If the 14C level in a sample of organic matter has been reduced to 0.200% of its original value, approximately how much time has passed? Radioactive decay follows first-order kinetics.
a. 1650 years
b. 51,400 years
c. 29,900 years
d 2,870,000 years
e. 9220 years

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
The half-life of radioactive carbon-14 is 5730 years. If the 14C level in a sample of organic matter...
Questions





Mathematics, 12.08.2020 06:01

Chemistry, 12.08.2020 06:01


Biology, 12.08.2020 06:01


English, 12.08.2020 06:01








Mathematics, 12.08.2020 06:01


Chemistry, 12.08.2020 06:01