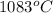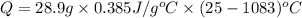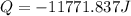Chemistry, 29.12.2019 18:31 jeffhuffle17
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 1083 degrees celsius to 25.0 degrees celsius. the specific heat of copper is .385 j/g celsius.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 10...
Questions

Mathematics, 08.12.2020 01:00

History, 08.12.2020 01:00







History, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

History, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00

Mathematics, 08.12.2020 01:00


Arts, 08.12.2020 01:00


Mathematics, 08.12.2020 01:00

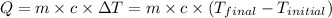
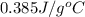
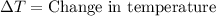
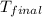 = final temperature =
= final temperature = 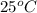
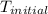 = initial temperature =
= initial temperature =