
Chemistry, 02.04.2020 22:14 bobbyhill24
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 -->2KCL + 3O2 How many moles of oxygen are formed?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1
You know the right answer?
A 7.11g sample of potassium chlorate was decomposed according to the following equation: 2KCLO3 --&g...
Questions

History, 17.05.2021 19:10

Business, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10

Health, 17.05.2021 19:10


Social Studies, 17.05.2021 19:10


Spanish, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10




Mathematics, 17.05.2021 19:10


Mathematics, 17.05.2021 19:10

Arts, 17.05.2021 19:10



Mathematics, 17.05.2021 19:10


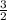 × 0.0580 moles
× 0.0580 moles

