The different combinations of cations and anions created by the student is 1 point
recorded in...

Chemistry, 02.04.2020 06:29 gsVKJCGAISGF46661
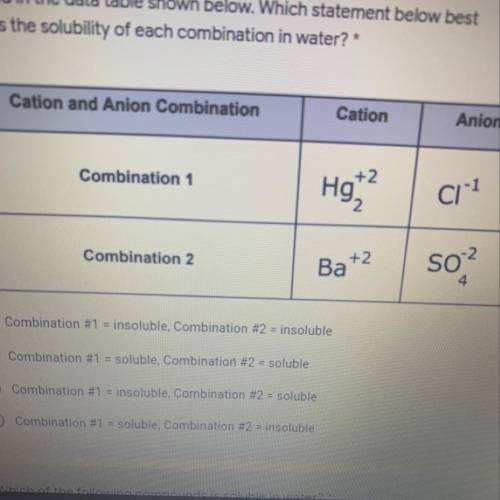
The different combinations of cations and anions created by the student is 1 point
recorded in the data table shown below. Which statement below best
predicts the solubility of each combination in water
A. Combination #1 = insoluble, Combination #2 = insoluble
B. Combination #1 = soluble, Combination #2 = soluble
C. Combination #1 = insoluble, Combination #2 = soluble
D. Combination #1 = soluble, Combination #2 = insoluble

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Questions

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01



Mathematics, 22.10.2020 04:01




Mathematics, 22.10.2020 04:01

History, 22.10.2020 04:01

Spanish, 22.10.2020 04:01



Arts, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01



Biology, 22.10.2020 04:01

Social Studies, 22.10.2020 04:01

Chemistry, 22.10.2020 04:01



