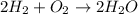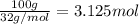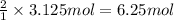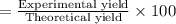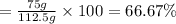Chemistry, 01.04.2020 21:45 mariarodriguezout9cj
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen gas are used, what would the percent yield be if 75 g of H2O was produced? Show your work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Consider the chemical equation for the production of water: 2 H2+O2→2 H2O. If 100 grams of oxygen ga...
Questions



English, 28.07.2021 07:00

Mathematics, 28.07.2021 07:00


Chemistry, 28.07.2021 07:00


Mathematics, 28.07.2021 07:00





Mathematics, 28.07.2021 07:00



Chemistry, 28.07.2021 07:00


Mathematics, 28.07.2021 07:00


Computers and Technology, 28.07.2021 07:00