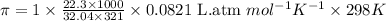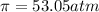Chemistry, 01.04.2020 19:31 kjjackson012002
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was added to water to make 321 mL of solution at 25.0 °C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
What is the osmotic pressure of a solution made from 22.3 g of methanol (MM = 32.04 g/mol) that was...
Questions







Mathematics, 17.12.2021 02:30





Mathematics, 17.12.2021 02:30

Social Studies, 17.12.2021 02:30


Mathematics, 17.12.2021 02:30

Mathematics, 17.12.2021 02:30


Mathematics, 17.12.2021 02:30


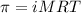
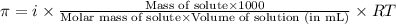
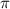 = osmotic pressure of the solution = ?
= osmotic pressure of the solution = ?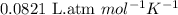
![25^oC=[273+25]=298K](/tpl/images/0575/9078/6a9f9.png)