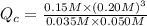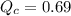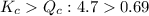Chemistry, 01.04.2020 19:33 doralisaponte79851
The first step in the industrial synthesis of hydrogen is the reaction of steam and methane to give synthesis gas, a mixture of carbon monoxide and hydrogen. h2o(g)+ch4(g)⇌co(g)+3h2(g) kc = 4.7 at 1400k. a mixture of reactants and products at 1400k contains 0.035 m h2o, 0.050m ch4, 0.15 m co, and 0.20 m h2.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1


Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
The first step in the industrial synthesis of hydrogen is the reaction of steam and methane to give...
Questions

Mathematics, 16.12.2021 14:00

Biology, 16.12.2021 14:00

English, 16.12.2021 14:00



World Languages, 16.12.2021 14:00

Mathematics, 16.12.2021 14:00


Social Studies, 16.12.2021 14:00


Mathematics, 16.12.2021 14:00


Physics, 16.12.2021 14:00



Computers and Technology, 16.12.2021 14:00

History, 16.12.2021 14:00

Social Studies, 16.12.2021 14:00


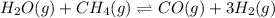
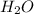 , 0.050 M
, 0.050 M 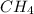 , 0.15 M
, 0.15 M 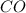 , and 0.20 M
, and 0.20 M 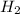 .
.
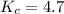
![[H_2O]=0.035 M](/tpl/images/0575/9166/507ff.png)
![[CH_4]=0.050 M](/tpl/images/0575/9166/de553.png)
![[CO]=0.15 M](/tpl/images/0575/9166/629de.png)
![[H_2]=0.20 M](/tpl/images/0575/9166/1d49e.png)
![Q_c=\frac{[CO][H_2]^3}{[H_2O][CH_4]}](/tpl/images/0575/9166/7c8ad.png)