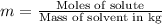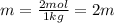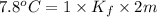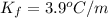Chemistry, 01.04.2020 18:36 kwarwick0915
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freezes at 7.8°c below its normal freezing point. what is the molal freezing-point constant of the unknown solvent? suggest a possible identity of the solvent.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Two moles of a nonelectrolyte solute are dissolved in 1 kg of an unknown solvent. the solution freez...
Questions



English, 23.01.2021 23:40


Mathematics, 23.01.2021 23:40



Law, 23.01.2021 23:40





Mathematics, 23.01.2021 23:40


Chemistry, 23.01.2021 23:40


Biology, 23.01.2021 23:40

English, 23.01.2021 23:40

Mathematics, 23.01.2021 23:40

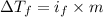
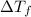 =depression in freezing point =
=depression in freezing point = 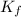 = freezing point constant
= freezing point constant