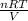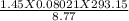Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
A gas sample occupies 8.77 L at 20 degrees Celsius. What is the pressure in atm, given that there ar...
Questions

Mathematics, 06.11.2020 14:00

History, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00


English, 06.11.2020 14:00

Spanish, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00

Computers and Technology, 06.11.2020 14:00


History, 06.11.2020 14:00

Physics, 06.11.2020 14:00

Social Studies, 06.11.2020 14:00


Mathematics, 06.11.2020 14:00

English, 06.11.2020 14:00

History, 06.11.2020 14:00


Social Studies, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00