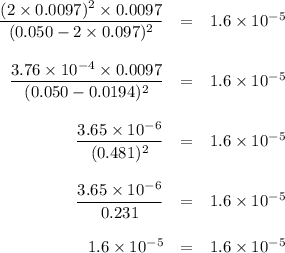Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
2NOCI (g)
→ 2NO(g) + Cl2 (g)
Problem:
Initially
the above reaction contains<...
→ 2NO(g) + Cl2 (g)
Problem:
Initially
the above reaction contains<...
Questions

Mathematics, 15.02.2020 00:17






Social Studies, 15.02.2020 00:17

English, 15.02.2020 00:17

Mathematics, 15.02.2020 00:18

English, 15.02.2020 00:18

Mathematics, 15.02.2020 00:18



Computers and Technology, 15.02.2020 00:18

Computers and Technology, 15.02.2020 00:18


Medicine, 15.02.2020 00:18

Mathematics, 15.02.2020 00:18


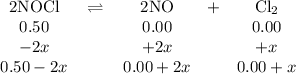
![K_{\text{c}} = \dfrac{\text{[NO]$^{2}$[Cl$_{2}$]}}{\text{[NOCl]}^{2}} = \dfrac{(2x)^{2}(x)}{(0.50 - 2x)^{2}} = 1.6 \times 10^{-5}\\\\4x^{3} = 1.6 \times 10^{-5}(0.50 - 2x)^{2}\\x^{3} = 4.0 \times 10^{-6}(0.50 - 2x)^{2}](/tpl/images/0575/0584/940ed.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50)^{2}} = 0.010](/tpl/images/0575/0584/0f7f4.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50 - 2\times 0.010)^{2}} = 0.0097](/tpl/images/0575/0584/39746.png)
![x = \sqrt [3] {4.0 \times 10^{-6}(0.50 - 2\times 0.0097)^{2}} = 0.0097](/tpl/images/0575/0584/5e7db.png)