
Chemistry, 01.04.2020 01:04 ericadawn2852
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reactant in the test tube and finds the mass to be 12.5g. He carries out a reaction by heating the test tube. At the end of the reaction, he measures the mass of the test tube containing the lone product and finds it to have a mass of 9.3g. What is the percent yield for this experiment if he expected to produce 5g of product?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
William measures a test tube and finds that the mass of the test tube is 5g. He places the lone reac...
Questions


Health, 13.11.2020 17:10


History, 13.11.2020 17:10




Mathematics, 13.11.2020 17:10

Social Studies, 13.11.2020 17:10


SAT, 13.11.2020 17:10









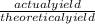 x 100
x 100 x 100
x 100

