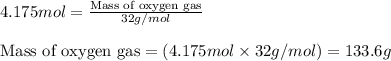Chemistry, 31.03.2020 21:25 mckenziew6969
4 NH3 + 5 O2 > 4 NO + 6 H2O
How many moles and how many grams of oxygen (O2) are needed to react with 56.8 grams
of ammonia by this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1

You know the right answer?
4 NH3 + 5 O2 > 4 NO + 6 H2O
How many moles and how many grams of oxygen (O2) are needed to...
How many moles and how many grams of oxygen (O2) are needed to...
Questions

History, 05.05.2020 05:20


English, 05.05.2020 05:20


Mathematics, 05.05.2020 05:20







History, 05.05.2020 05:20

Mathematics, 05.05.2020 05:20


Mathematics, 05.05.2020 05:20


Mathematics, 05.05.2020 05:20

Mathematics, 05.05.2020 05:20

Biology, 05.05.2020 05:20

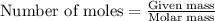 .....(1)
.....(1)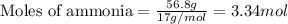
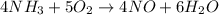
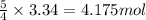 of oxygen gas
of oxygen gas