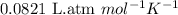Chemistry, 31.03.2020 19:35 robert7248
PLEASE ANSWER ASAP AND SHOW WORK: A camping stove uses a 5.0 L propane tank that holds 68.0 moles of liquid C3H8. How large a container would be needed to hold the same amount pf propane as a gas at 25.0 ⁰C and a pressure of 3.0 atm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
PLEASE ANSWER ASAP AND SHOW WORK: A camping stove uses a 5.0 L propane tank that holds 68.0 moles of...
Questions



History, 04.05.2021 21:30





Engineering, 04.05.2021 21:30


Physics, 04.05.2021 21:30



Arts, 04.05.2021 21:30

History, 04.05.2021 21:30

Mathematics, 04.05.2021 21:30


Mathematics, 04.05.2021 21:30


Mathematics, 04.05.2021 21:30

History, 04.05.2021 21:30

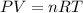
![25^oC=[25+273]K=298K](/tpl/images/0573/6257/df1f6.png)