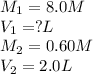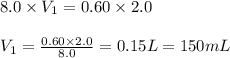Chemistry, 31.03.2020 19:17 BakedBiscuit6896
A student needs to prepare 2.0 liters 0.60 M NaOH solution from a 8.0 M NaOH stock solution. How many milliliters of stock solution should the student use?(1 point)
A.150 mL
B.225 ml
C.250 mL
D.275 mL

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
A student needs to prepare 2.0 liters 0.60 M NaOH solution from a 8.0 M NaOH stock solution. How man...
Questions


Mathematics, 18.03.2021 06:00

Social Studies, 18.03.2021 06:00

Mathematics, 18.03.2021 06:00

Mathematics, 18.03.2021 06:10


Mathematics, 18.03.2021 06:10





English, 18.03.2021 06:10

Mathematics, 18.03.2021 06:10



World Languages, 18.03.2021 06:10

Physics, 18.03.2021 06:10

Physics, 18.03.2021 06:10


Geography, 18.03.2021 06:10

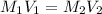
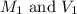 are the molarity and volume of the stock NaOH solution
are the molarity and volume of the stock NaOH solution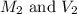 are the molarity and volume of diluted NaOH solution
are the molarity and volume of diluted NaOH solution