HELP PLEASE
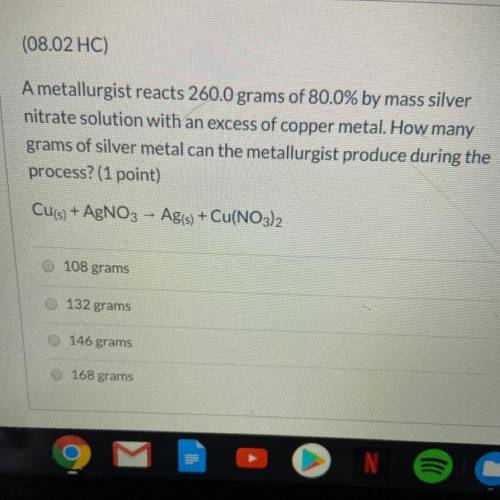
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution w...

Chemistry, 31.03.2020 18:56 sparkyjones02
HELP PLEASE
A metallurgist reacts 260.0 grams of 80.0% by mass silver nitrate solution with an excess of copper metal. How many grams of silver metal can the metallurgist produce during the process?
Cu(s) + AgNO3 - Ag(s) + Cu(NO3)2
Answers
A.108 grams
B.132 grams
C.146 grams
D.168 grams

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 2



Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1
You know the right answer?
Questions







Mathematics, 30.11.2019 05:31


Mathematics, 30.11.2019 05:31

SAT, 30.11.2019 05:31

Mathematics, 30.11.2019 05:31


Mathematics, 30.11.2019 05:31


Computers and Technology, 30.11.2019 05:31

Chemistry, 30.11.2019 05:31

History, 30.11.2019 05:31

Geography, 30.11.2019 05:31





