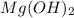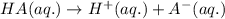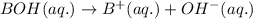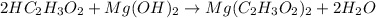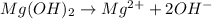2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 23.06.2019 10:10
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2

You know the right answer?
Identify the base in this acid-base reaction:
2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O
2HC2H3O2 + Mg(OH)2 = Mg(C2H3O2)2 + 2H2O
Questions


Mathematics, 06.10.2020 01:01


Mathematics, 06.10.2020 01:01






History, 06.10.2020 01:01


Chemistry, 06.10.2020 01:01


Mathematics, 06.10.2020 01:01

Physics, 06.10.2020 01:01


Chemistry, 06.10.2020 01:01

Mathematics, 06.10.2020 01:01

World Languages, 06.10.2020 01:01