

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

You know the right answer?
Starting with 9.3 moles of O2, how many moles of H2S will be needed and how many moles of SO2 will b...
Questions


English, 18.07.2019 07:30



Mathematics, 18.07.2019 07:30

Biology, 18.07.2019 07:30



Biology, 18.07.2019 07:30

Biology, 18.07.2019 07:30





History, 18.07.2019 07:30

Chemistry, 18.07.2019 07:30


History, 18.07.2019 07:30

Mathematics, 18.07.2019 07:30

Chemistry, 18.07.2019 07:30

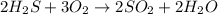
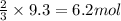 of hydrogen sulfide
of hydrogen sulfide

