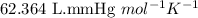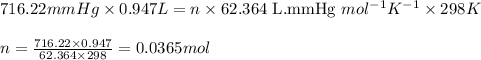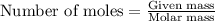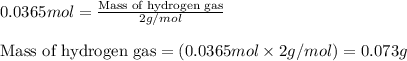Chemistry, 31.03.2020 17:11 hhomeschool24
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). The reaction between the acid and the zinc is as follows: 2H+(aq)+Zn(s)→H2(g)+Zn2+(aq). When the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘C was 0.947 L at a total pressure of 740 mmHg. What mass of hydrogen gas is collected?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions

Physics, 09.02.2021 18:40


Computers and Technology, 09.02.2021 18:40

Mathematics, 09.02.2021 18:40




Mathematics, 09.02.2021 18:40

English, 09.02.2021 18:40

Mathematics, 09.02.2021 18:40



English, 09.02.2021 18:40

History, 09.02.2021 18:40

Mathematics, 09.02.2021 18:40

Mathematics, 09.02.2021 18:40




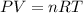
![25^oC=[25+273]K=298K](/tpl/images/0573/2769/df1f6.png)