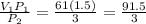Chemistry, 31.03.2020 14:08 itsyogirl12
A Sample of helium measuring 6l was kept at a pressure of 1.5atm. If pressure is doubled what would be its new volume?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
A Sample of helium measuring 6l was kept at a pressure of 1.5atm. If pressure is doubled what would...
Questions

History, 01.08.2019 01:00



Health, 01.08.2019 01:00



History, 01.08.2019 01:00


English, 01.08.2019 01:00

History, 01.08.2019 01:00

Business, 01.08.2019 01:00

History, 01.08.2019 01:00




History, 01.08.2019 01:00