

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
The natural distribution of the isotopes of a hypothetical element is 60.795% at a mass of 281.99481...
Questions


History, 31.05.2021 20:10





Mathematics, 31.05.2021 20:10

Business, 31.05.2021 20:10

Biology, 31.05.2021 20:10



English, 31.05.2021 20:10




English, 31.05.2021 20:10




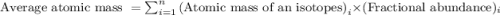 .....(1)
.....(1)![\text{Average atomic mass of element}=[(281.99481\times 0.60795)+(283.99570\times 0.22122)+(286.99423\times 0.17083)]\\\\\text{Average atomic mass of element}=283.291amu](/tpl/images/0572/8936/1f7da.png)


