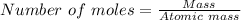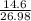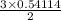Chemistry, 31.03.2020 04:37 chantianabess36
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+3H2 (g)2Al(s)+3H2SO4(aq)→Al2(SO4)3(aq)+ 3H2(g) Suppose you wanted to dissolve an aluminum block with a mass of 14.6 gg . Part A What minimum mass of H2SO4H2SO4 would you need? Express your answer in grams.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Sulfuric acid dissolves aluminum metal according to the following reaction: 2Al(s)+3H2SO4(aq)→Al2(SO...
Questions


Mathematics, 31.10.2020 01:00


Chemistry, 31.10.2020 01:00



Mathematics, 31.10.2020 01:00



Mathematics, 31.10.2020 01:00


Physics, 31.10.2020 01:00

Computers and Technology, 31.10.2020 01:00


Biology, 31.10.2020 01:00





Mathematics, 31.10.2020 01:00