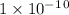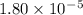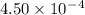Chemistry, 31.03.2020 04:19 jessicaflower277
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H Ka = 1.80 × 10-5 Nitrous acid, HNO2 Ka = 4.50 × 10-4 What is the order of increasing base strength for C6H5O−, CH3COO−, and NO2−? Group of answer choices

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1
You know the right answer?
Given the following acids and their Ka values: Phenol, C6H5OH Ka = 1.00 × 10-10 Acetic acid, CH3CO2H...
Questions


History, 11.12.2021 22:40


History, 11.12.2021 22:40

Mathematics, 11.12.2021 22:40



Mathematics, 11.12.2021 22:40


Mathematics, 11.12.2021 22:50

Mathematics, 11.12.2021 22:50


Geography, 11.12.2021 22:50

Mathematics, 11.12.2021 22:50


Mathematics, 11.12.2021 22:50

Mathematics, 11.12.2021 22:50



Biology, 11.12.2021 22:50

 <
< 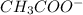 <
< 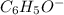
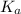 value is a measure of acidic strength. The larger the
value is a measure of acidic strength. The larger the