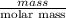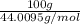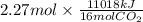Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Using the following equation for the combustion of octane, calculate the heat associated with the fo...
Questions




Health, 31.07.2019 10:20

Mathematics, 31.07.2019 10:20






Physics, 31.07.2019 10:20

Health, 31.07.2019 10:20


Physics, 31.07.2019 10:20

Mathematics, 31.07.2019 10:20

Mathematics, 31.07.2019 10:20



Business, 31.07.2019 10:20

Biology, 31.07.2019 10:20

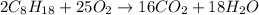 ;
; 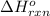 = -11018 kJ
= -11018 kJ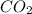 = 100 g
= 100 g