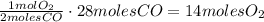Chemistry, 31.03.2020 02:59 camiserjai1832
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emissions such as carbon monoxide, CO(g), into carbon dioxide, CO2(g). The uncatalyzed reaction is represented by the balanced equation below.2CO(g) O2(g) 2CO2(g) heatDetermine the number of moles of O2(g) required to completely react with 28 moles of CO(g) during this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Describe in detail the melting point behavior of the 80: 20 benzoic acid-mandelic acid mixture
Answers: 3

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Automobile catalytic converters use a platinum catalyst to reduce air pollution by changing emission...
Questions


Mathematics, 24.04.2020 23:49

History, 24.04.2020 23:49

English, 24.04.2020 23:49


Mathematics, 24.04.2020 23:49

Mathematics, 24.04.2020 23:49

Mathematics, 24.04.2020 23:49






History, 24.04.2020 23:50


Geography, 24.04.2020 23:50



Mathematics, 24.04.2020 23:50