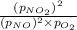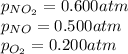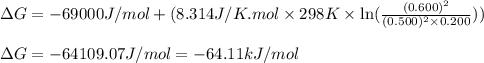For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all species. For the reaction 2 NO ( g ) + O 2 ( g ) − ⇀ ↽ − 2 NO 2 ( g ) the standard change in Gibbs free energy is Δ G ° = − 69.0 kJ/mol . What is ΔG for this reaction at 298 K when the partial pressures are P NO = 0.500 atm , P O 2 = 0.200 atm , and P NO 2 = 0.600 atm ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
For a gaseous reaction, standard conditions are 298 K and a partial pressure of 1 atm for all specie...
Questions

Mathematics, 26.11.2020 08:00

English, 26.11.2020 08:00

Mathematics, 26.11.2020 08:10

Mathematics, 26.11.2020 08:10


History, 26.11.2020 08:10

English, 26.11.2020 08:10

German, 26.11.2020 08:10



History, 26.11.2020 08:10




Computers and Technology, 26.11.2020 08:20



Social Studies, 26.11.2020 08:20

Social Studies, 26.11.2020 08:20

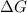 for the given reaction at 298 K is -64.11 kJ/mol
for the given reaction at 298 K is -64.11 kJ/mol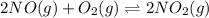
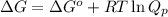
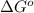 = Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Standard Gibbs free energy = -69.0 kJ/mol = -69000 J/mol (Conversion factor: 1 kJ = 1000 J)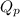 = Ratio of partial pressure of products and reactants =
= Ratio of partial pressure of products and reactants =