Chemistry, 31.03.2020 02:17 untouchedyannaa
Multiple-Concept Example 3 reviews the concepts necessary to solve this problem. Radiation with a wavelength of 238 nm shines on a metal surface and ejects electrons that have a maximum speed of 3.75 × 105 m/s. Which one of the following metals is it, the values in parentheses being the work functions: potassium (2.24 eV), calcium (2.71 eV), uranium (3.63 eV), aluminum (4.08 eV), or gold (4.82 eV)?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

You know the right answer?
Multiple-Concept Example 3 reviews the concepts necessary to solve this problem. Radiation with a wa...
Questions

Geography, 27.08.2019 13:20

Arts, 27.08.2019 13:20





History, 27.08.2019 13:20


Mathematics, 27.08.2019 13:20



History, 27.08.2019 13:20

Mathematics, 27.08.2019 13:20


Geography, 27.08.2019 13:30


Mathematics, 27.08.2019 13:30



Mathematics, 27.08.2019 13:30

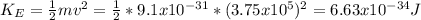 = 0.4 eV
= 0.4 eV