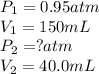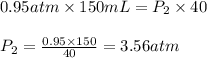Chemistry, 31.03.2020 01:30 mjstew00763
A sample of helium gas has a volume of 150. Ml (V₁) at 0.95 atom (P₁). What will your new pressure (P₁) be if the volume is changed to 40.0 ml (V₁) and temperature remains constant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
A sample of helium gas has a volume of 150. Ml (V₁) at 0.95 atom (P₁). What will your new pressure (...
Questions

Mathematics, 18.10.2021 21:20


Physics, 18.10.2021 21:20


English, 18.10.2021 21:20

Mathematics, 18.10.2021 21:20

Social Studies, 18.10.2021 21:20



History, 18.10.2021 21:20


English, 18.10.2021 21:20

History, 18.10.2021 21:20


Mathematics, 18.10.2021 21:20

History, 18.10.2021 21:20

Mathematics, 18.10.2021 21:20



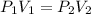
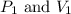 are initial pressure and volume.
are initial pressure and volume.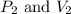 are final pressure and volume.
are final pressure and volume.