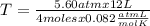Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
At what temperature in K will 4.00 moles of gas occupy a volume of 12.0 L at a pressure of 5.60 atm?...
Questions

Social Studies, 09.11.2019 02:31




English, 09.11.2019 02:31

Biology, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31

English, 09.11.2019 02:31

Biology, 09.11.2019 02:31


Mathematics, 09.11.2019 02:31


History, 09.11.2019 02:31

History, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31

Mathematics, 09.11.2019 02:31


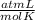 T=?
T=?