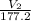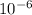Chemistry, 31.03.2020 01:01 victoria02117
Starting with a vessel of temperature 50.1°C, a pressure of 6263.MmHg and volume 461.1mL, what is its final volume, in liters, if you cool it to -95.8°C with a final pressure of 411.Atm?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Starting with a vessel of temperature 50.1°C, a pressure of 6263.MmHg and volume 461.1mL, what is it...
Questions

Computers and Technology, 26.10.2021 02:20








Mathematics, 26.10.2021 02:20

World Languages, 26.10.2021 02:20

Mathematics, 26.10.2021 02:20

English, 26.10.2021 02:20

Mathematics, 26.10.2021 02:20

Mathematics, 26.10.2021 02:20


Computers and Technology, 26.10.2021 02:20



Mathematics, 26.10.2021 02:20

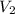 = 5.17 ml
= 5.17 ml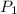 = 6263 mm Hg = 8.24 atm = 835 K pa
= 6263 mm Hg = 8.24 atm = 835 K pa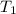 = 50.1 ° c = 323.1 K
= 50.1 ° c = 323.1 K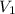 = 461.1 ml = 0.0004611
= 461.1 ml = 0.0004611 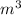
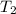 = - 95.8 ° c = 177.2 K
= - 95.8 ° c = 177.2 K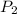 = 411 atm = 41644.6 K PA
= 411 atm = 41644.6 K PA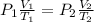
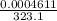 = 41644.6 ×
= 41644.6 ×