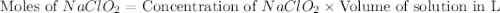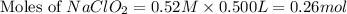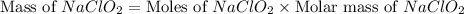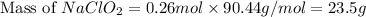Chemistry, 31.03.2020 01:01 nestergurl101
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid is a weak acid with =Ka×1.110−2. What mass of NaClO2 should the student dissolve in the HClO2 solution to turn it into a buffer with pH =2.11?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2
You know the right answer?
A chemistry graduate student is given 500.mL of a 0.40M chlorous acid HClO2 solution. Chlorous acid...
Questions

English, 03.06.2021 09:20

Mathematics, 03.06.2021 09:20

Physics, 03.06.2021 09:20

SAT, 03.06.2021 09:20



Mathematics, 03.06.2021 09:20


Mathematics, 03.06.2021 09:20





Health, 03.06.2021 09:20

Mathematics, 03.06.2021 09:20




Biology, 03.06.2021 09:20

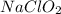 should be, 23.5 grams.
should be, 23.5 grams.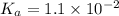
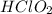 = 0.40 M
= 0.40 M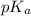 .
.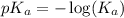
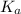 in this expression, we get:
in this expression, we get: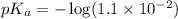
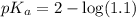
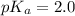
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0572/2296/e961a.png)
![pH=pK_a+\log \frac{[NaClO_2]}{[HClO_2]}](/tpl/images/0572/2296/a8df0.png)
![2.11=2.0+\log (\frac{[NaClO_2]}{0.40})](/tpl/images/0572/2296/3a0b0.png)
![[NaClO_2]=0.52M](/tpl/images/0572/2296/2adfa.png)