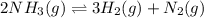Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Consider the following system at equilibrium at 723 K: 2 NH3 (g) 26.6 kcal N2 (g) 3 H2 (g) Indicate...
Questions

Mathematics, 29.05.2020 06:02


Biology, 29.05.2020 06:02



Mathematics, 29.05.2020 06:02

Mathematics, 29.05.2020 06:02



English, 29.05.2020 06:02





Chemistry, 29.05.2020 06:02

Mathematics, 29.05.2020 06:02

Mathematics, 29.05.2020 06:02

Biology, 29.05.2020 06:02

Health, 29.05.2020 06:02

Physics, 29.05.2020 06:02