

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
If the equilibrium constant for the reaction 3I- (aq) + S2O82- (aq) I3- (aq) + 2SO42- (aq) is K, w...
Questions

History, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01


Arts, 18.10.2020 01:01

Mathematics, 18.10.2020 01:01



English, 18.10.2020 01:01



Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01


Mathematics, 18.10.2020 01:01

Chemistry, 18.10.2020 01:01

![K'=\sqrt[3]{\frac{1}{K}}](/tpl/images/0572/2471/9553b.png)
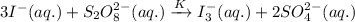 ......(1)
......(1)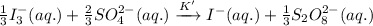 .........(2)
.........(2)

