
Chemistry, 31.03.2020 00:47 KindaSmartPersonn
A chemist prepares a solution of ironIII chloride FeCl3 by measuring out 72.7mg of FeCl3 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl− anions in the chemist's solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

You know the right answer?
A chemist prepares a solution of ironIII chloride FeCl3 by measuring out 72.7mg of FeCl3 into a 100....
Questions








English, 27.07.2021 17:20

Mathematics, 27.07.2021 17:20


Computers and Technology, 27.07.2021 17:20





Spanish, 27.07.2021 17:20

Mathematics, 27.07.2021 17:20

Biology, 27.07.2021 17:20


Biology, 27.07.2021 17:20

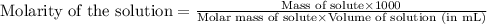
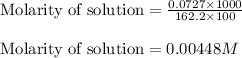
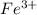 ions and 3 moles of
ions and 3 moles of 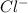 ions
ions


