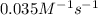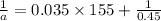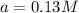Chemistry, 31.03.2020 00:42 marieknight689
The reaction X → products is second order in X and has a rate constant of 0.035 M−1s−1. If a reaction mixture is initially 0.45 M in X, what is the concentration of X after 155 seconds?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
The reaction X → products is second order in X and has a rate constant of 0.035 M−1s−1. If a reactio...
Questions

Mathematics, 14.10.2019 09:10



Chemistry, 14.10.2019 09:10

Social Studies, 14.10.2019 09:10

Physics, 14.10.2019 09:10

Mathematics, 14.10.2019 09:10

Mathematics, 14.10.2019 09:10






History, 14.10.2019 09:10


Mathematics, 14.10.2019 09:10


English, 14.10.2019 09:10

Biology, 14.10.2019 09:10

Mathematics, 14.10.2019 09:10

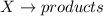
![rate=k[X]^2](/tpl/images/0572/1335/75d95.png)
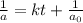
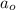 = initial concentration = 0.45 M
= initial concentration = 0.45 M