

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3


Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
If you combine 250.0 mL 250.0 mL of water at 25.00 ∘ C 25.00 ∘C and 100.0 mL 100.0 mL of water at 95...
Questions

Social Studies, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30


English, 08.07.2019 16:30

Social Studies, 08.07.2019 16:30



Biology, 08.07.2019 16:30




History, 08.07.2019 16:30

Social Studies, 08.07.2019 16:30

Computers and Technology, 08.07.2019 16:30

Business, 08.07.2019 16:30

Biology, 08.07.2019 16:30

Biology, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

Social Studies, 08.07.2019 16:30

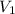 = 250 ml
= 250 ml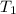 = 25 °c
= 25 °c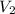 = 100 ml
= 100 ml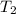 = 95 °c
= 95 °c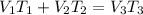
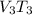
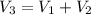
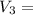 250 + 100 = 350 ml
250 + 100 = 350 ml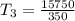
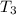 = 45 °c
= 45 °c

