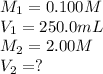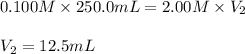Chemistry, 31.03.2020 00:27 shaymabejja1965
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solution of 2.00 M copper (II) chloride is available. How much of the stock solution is needed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
In an experiment, a student needs 250.0 mL of a 0.100 M copper (II) chloride solution. A stock solut...
Questions

Advanced Placement (AP), 19.01.2021 23:30


Biology, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

French, 19.01.2021 23:30


Mathematics, 19.01.2021 23:30






English, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30



English, 19.01.2021 23:30

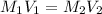
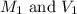 are the initial molarity and volume of copper (II) chloride.
are the initial molarity and volume of copper (II) chloride.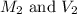 are the final molarity and volume of stock solution of copper (II) chloride.
are the final molarity and volume of stock solution of copper (II) chloride.