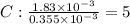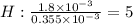Chemistry, 31.03.2020 00:27 CatsandDogsaredabest
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combustion of 29.5 mg produced 80.1 mg of CO2 and 16.4 mg of H2O. The molar mass of the compound was 162 g/mol. Determine its empirical and molecular formulas.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1


You know the right answer?
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combu...
Questions




Mathematics, 27.11.2019 07:31




Mathematics, 27.11.2019 07:31


Social Studies, 27.11.2019 07:31

Mathematics, 27.11.2019 07:31




Chemistry, 27.11.2019 07:31


Chemistry, 27.11.2019 07:31


History, 27.11.2019 07:31

Mathematics, 27.11.2019 07:31

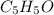 .
.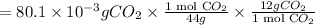
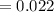 g
g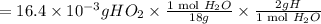
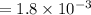 g
g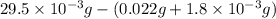
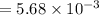 g
g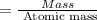
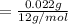
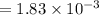 mol
mol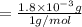
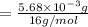
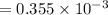 mol
mol