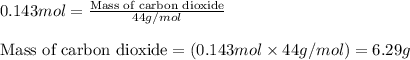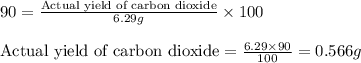Chemistry, 31.03.2020 00:15 erinleyanne
Consider the balanced equation for the following reaction:
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(l)
Determine the amount of CO2(g) formed in the reaction if 8.00 grams of O2(g) reacts with an excess of C2H6(g) and the percent yield of CO2(g) is 90.0%.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1


Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2
You know the right answer?
Consider the balanced equation for the following reaction:
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
7O2(g) + 2C2H6(g) → 4CO2(g) + 6H2O(...
Questions

Physics, 13.09.2020 16:01

English, 13.09.2020 16:01

English, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

History, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

English, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Biology, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

 .......(1)
.......(1)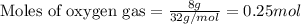
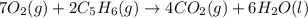
 of carbon dioxide
of carbon dioxide