
Chemistry, 31.03.2020 00:14 aubreyfoster
Express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
a. rate=12Δ[HBr]Δt=−Δ[H2]Δt=−Δ[Br2]Δtr ate=12Δ[HBr]Δt=−Δ[H2]Δt=−Δ[Br2]Δt
b. rate=−Δ[HBr]Δt=12Δ[H2]Δt=12Δ[Br2]Δt rate=−Δ[HBr]Δt=12Δ[H2]Δt=12Δ[Br2]Δt
c. rate=−12Δ[HBr]Δt=Δ[H2]Δt=Δ[Br2]Δtra te=−12Δ[HBr]Δt=Δ[H2]Δt=Δ[Br2]Δt
d. rate=Δ[HBr]Δt=−12Δ[H2]Δt=−12Δ[Br2]Δ t

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 23.06.2019 19:40
Can someone explain why the rangers are doing so bad this year
Answers: 1
You know the right answer?
Express the rate of the reaction in terms of the change in concentration of each of the reactants an...
Questions

Mathematics, 16.12.2021 05:30




Mathematics, 16.12.2021 05:30

Social Studies, 16.12.2021 05:30




Physics, 16.12.2021 05:30



Mathematics, 16.12.2021 05:30

English, 16.12.2021 05:30

Mathematics, 16.12.2021 05:30

English, 16.12.2021 05:30

Mathematics, 16.12.2021 05:30



Mathematics, 16.12.2021 05:30

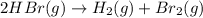
![rate=-\frac{1}{2} \frac{\Delta [HBr]}{\Delta t}=\frac{\Delta [Br_2]}{\Delta t} =\frac{\Delta [H_2]}{\Delta t}](/tpl/images/0572/0368/fc117.png)


