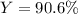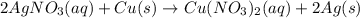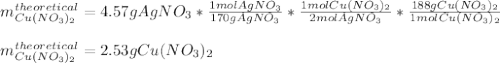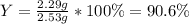For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yields 2.29 gram of copper(II) nitrate What is the percent yield for this reaction?Formula: % yield = (Actual yield/theoretical yield) x 100 2 AgNO3(aq) + Cu(s) à Cu(NO3)2 (aq) + 2 Ag(s)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yiel...
Questions


Arts, 03.12.2020 19:00

Mathematics, 03.12.2020 19:00

Biology, 03.12.2020 19:00


History, 03.12.2020 19:00




Mathematics, 03.12.2020 19:00

English, 03.12.2020 19:00

Mathematics, 03.12.2020 19:00

Business, 03.12.2020 19:00

Mathematics, 03.12.2020 19:00


History, 03.12.2020 19:00


Mathematics, 03.12.2020 19:00

English, 03.12.2020 19:00