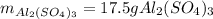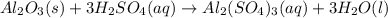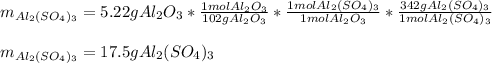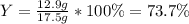Chemistry, 30.03.2020 23:55 guadalupemarlene2001
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The reaction yields 12.9 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) What is the theoretical yield of aluminum sulfate ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1
You know the right answer?
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The re...
Questions

Physics, 06.10.2019 00:00



Mathematics, 06.10.2019 00:00







Social Studies, 06.10.2019 00:00

Mathematics, 06.10.2019 00:00



History, 06.10.2019 00:00


Social Studies, 06.10.2019 00:00