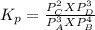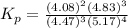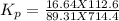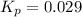At 25 ∘ C , the equilibrium partial pressures for the reaction 3 A ( g ) + 4 B ( g ) − ⇀ ↽ − 2 C ( g ) + 3 D ( g ) were found to be P A = 4.47 atm, P B = 5.17 atm, P C = 4.08 atm, and P D = 4.83 atm. What is the standard change in Gibbs free energy of this reaction at 25 ∘ C ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
At 25 ∘ C , the equilibrium partial pressures for the reaction 3 A ( g ) + 4 B ( g ) − ⇀ ↽ − 2 C ( g...
Questions

Mathematics, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20



Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

English, 23.04.2021 20:20


English, 23.04.2021 20:20


Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

Mathematics, 23.04.2021 20:20

History, 23.04.2021 20:20