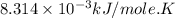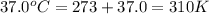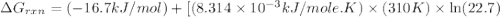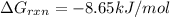Chemistry, 31.03.2020 00:01 samueltaye
Calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 22.7 and the temperature is 37.0 ° C ? Δ G ° ' for the reaction is − 16.7 kJ/mol .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
Calculate the free energy change if the ratio of the concentrations of the products to the concentra...
Questions



Computers and Technology, 08.03.2021 06:50



Mathematics, 08.03.2021 06:50



Mathematics, 08.03.2021 06:50


Mathematics, 08.03.2021 06:50

Mathematics, 08.03.2021 06:50

English, 08.03.2021 06:50

World Languages, 08.03.2021 06:50

Mathematics, 08.03.2021 06:50

Computers and Technology, 08.03.2021 06:50


Arts, 08.03.2021 06:50


Social Studies, 08.03.2021 06:50

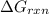 is -8.65 kJ/mol
is -8.65 kJ/mol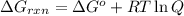 ............(1)
............(1)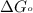 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol