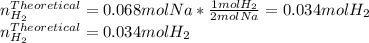Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
When sodium metal is added to water, the following reaction occurs: 2Na(s) + 2H2O(l) → 2NaOH(aq) + H...
Questions


Mathematics, 08.12.2021 04:20

Mathematics, 08.12.2021 04:20

Mathematics, 08.12.2021 04:20


History, 08.12.2021 04:20

Mathematics, 08.12.2021 04:20

Mathematics, 08.12.2021 04:20


English, 08.12.2021 04:20





Arts, 08.12.2021 04:20

Mathematics, 08.12.2021 04:20




Chemistry, 08.12.2021 04:20