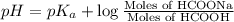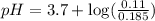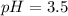Chemistry, 30.03.2020 23:33 erikamaldonado661
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100M sodium formate, HCOONa after the addition of 10.0 mL of 6.00M NaOH to the original buffered solution volume of 500.0 mL.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
Calculate the pH of a solution that contains 0.250 M formic acid, HCOOH (Ka =1.8 x 10-4 ), and 0.100...
Questions


Social Studies, 22.08.2019 09:50


History, 22.08.2019 09:50

Mathematics, 22.08.2019 09:50

Mathematics, 22.08.2019 09:50

Mathematics, 22.08.2019 09:50


Social Studies, 22.08.2019 10:00


Physics, 22.08.2019 10:00



Physics, 22.08.2019 10:00

Biology, 22.08.2019 10:00

Mathematics, 22.08.2019 10:00

Mathematics, 22.08.2019 10:00

Health, 22.08.2019 10:00

Mathematics, 22.08.2019 10:00

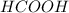 ,
, 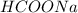 and
and 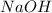 .
.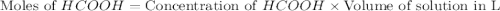
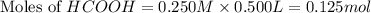
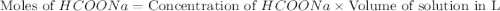
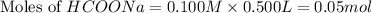
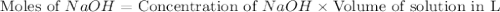
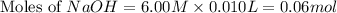
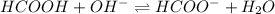
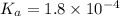
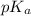 .
.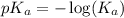
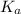 in this expression, we get:
in this expression, we get: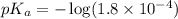
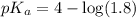
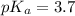
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0571/8655/e961a.png)
![pH=pK_a+\log \frac{[HCOONa]}{[HCOOH]}](/tpl/images/0571/8655/c5edc.png)
![pH=pK_a+\log \frac{[\frac{\text{Moles of HCOONa}}{\text{Volume of solution}}]}{[\frac{\text{Moles of HCOOH}}{\text{Volume of solution}}]}](/tpl/images/0571/8655/fba20.png)