
Chemistry, 30.03.2020 23:33 mathman783
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? 4.10 × 106 s-1 713 s-1 1.36 × 102 s-1 2.65 × 104 s-1 3.69 × 104 s-1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magni...
Questions

Health, 22.11.2020 22:30


Mathematics, 22.11.2020 22:30

Mathematics, 22.11.2020 22:30



Mathematics, 22.11.2020 22:30

English, 22.11.2020 22:30

Mathematics, 22.11.2020 22:30

Mathematics, 22.11.2020 22:30




Mathematics, 22.11.2020 22:30




Chemistry, 22.11.2020 22:30


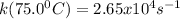
![\frac{k(75.0^0C)}{k(25.0^0C)} =exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]](/tpl/images/0571/8653/57013.png)
![k(75.0^0C)=k(25.0^0C)exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]\\\\k(75.0^0C)=1.35x10^2s^{-1}exp[-\frac{91000J/mol}{8.314J/(mol*K)}(\frac{1}{348.15K}-\frac{1}{298.15K} )]\\\\k(75.0^0C)=2.65 x 10^4 s^{-1}](/tpl/images/0571/8653/debb3.png)


